Online Database of Chemicals from Around the World
| Changzhou Jiuwu Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8512-1086 +86 13775162768 | |||
 |
info@jiuwuchem.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink premium supplier since 2016 | ||||
| Classification | Organic raw materials >> Alcohols, phenols, phenolic compounds and derivatives |
|---|---|
| Name | 1-Hydrazinylpropan-2-ol |
| Molecular Structure | 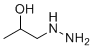 |
| Molecular Formula | C3H10N2O |
| Molecular Weight | 90.12 |
| CAS Registry Number | 18501-20-7 |
| EC Number | 833-904-6 |
| SMILES | CC(CNN)O |
| Density | 1.0±0.1 g/cm3 Calc.*, 1.019 g/mL (Expl.) |
|---|---|
| Boiling point | 244.9±23.0 ºC 760 mmHg (Calc.)*, 245 ºC (Expl.) |
| Flash point | 101.9±22.6 ºC (Calc.)*, 101.9 ºC (Expl.) |
| Index of refraction | 1.468 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS06;GHS07 Danger Details
GHS06;GHS07 Danger Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P316-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
|
1-Hydrazinylpropan-2-ol is a small aliphatic hydrazine derivative that contains both a hydrazinyl functional group and a secondary alcohol within a three-carbon backbone. Compounds combining hydrazine moieties with alcohol functionality occupy a niche position in organic and medicinal chemistry, primarily as reactive intermediates rather than end-use products. The chemical interest in 1-hydrazinylpropan-2-ol arises from the distinctive reactivity of hydrazines and their long-established role in the synthesis of nitrogen-containing molecules. The discovery and study of 1-hydrazinylpropan-2-ol are linked to broader investigations into hydrazine chemistry that began in the late nineteenth century. Hydrazine and its derivatives were initially explored for their reducing properties and their ability to form hydrazones and azines with carbonyl compounds. As organic synthesis evolved, substituted hydrazines became important building blocks for heterocycle formation, pharmaceutical intermediates, and functional materials. Alcohol-functionalized hydrazines such as 1-hydrazinylpropan-2-ol emerged as part of systematic efforts to diversify hydrazine structures and to control their solubility, reactivity, and handling characteristics. From a synthetic perspective, 1-hydrazinylpropan-2-ol is typically prepared through controlled substitution reactions starting from appropriately functionalized propanol derivatives. Common approaches involve the conversion of a leaving group, such as a halide or sulfonate, into a hydrazinyl substituent under nucleophilic conditions. Alternatively, reductive transformations of precursor hydrazones or related nitrogen-containing intermediates can yield the target compound. Careful control of reaction conditions is essential, as hydrazine derivatives are prone to side reactions and oxidation. The presence of the hydroxyl group can influence both the regioselectivity and the stability of intermediates during synthesis. The chemical behavior of 1-hydrazinylpropan-2-ol reflects the combined properties of its functional groups. The hydrazinyl moiety is strongly nucleophilic and can readily react with aldehydes, ketones, activated esters, and electrophilic heterocycles to form new carbon–nitrogen bonds. This reactivity underpins its usefulness as an intermediate in synthetic chemistry. The secondary alcohol group can participate in hydrogen bonding and may be further modified through oxidation, esterification, or ether formation. Together, these functionalities allow the molecule to serve as a flexible platform for multistep synthesis. In application-driven research, 1-hydrazinylpropan-2-ol has primarily been employed as a reagent or intermediate rather than as a final commercial product. Hydrazine-containing intermediates are widely used in the preparation of pharmaceuticals, agrochemicals, and fine chemicals, particularly in routes leading to heterocyclic compounds such as pyrazoles, triazoles, and related nitrogen-rich ring systems. The incorporation of a hydroxyl group can improve compatibility with polar solvents and facilitate downstream transformations, making compounds like 1-hydrazinylpropan-2-ol useful in laboratory-scale synthesis. In medicinal chemistry, hydrazine derivatives have historically been investigated for biological activity, including antimicrobial and enzyme-inhibitory properties. While 1-hydrazinylpropan-2-ol itself is not commonly reported as a therapeutic agent, its structural motif can be incorporated into more complex molecules during lead optimization or structure–activity relationship studies. In such contexts, the compound functions as a synthetic precursor that enables the introduction of hydrazine-based linkages or functional groups into target molecules. Safety considerations are an important aspect of working with 1-hydrazinylpropan-2-ol. As with many hydrazine derivatives, it requires careful handling due to potential toxicity and reactivity. These characteristics further reinforce its role as a controlled intermediate used by trained chemists rather than a substance intended for widespread application. Overall, 1-hydrazinylpropan-2-ol represents a small but chemically significant molecule whose importance lies in its utility within synthetic chemistry. Its development and use reflect the enduring relevance of hydrazine derivatives as tools for constructing nitrogen-containing compounds and exploring new chemical space in research and development. References 2014. Ratio of 1,3- and 1,5-dialkyl-substituted pyrazoles obtained from chlorovinyl alkyl ketones and alkylhydrazines, 3(5)-pyrazoles and alkyl bromides. Russian Journal of Organic Chemistry, 50(11). DOI: 10.1134/s1070428014110190 |
| Market Analysis Reports |
| List of Reports Available for 1-Hydrazinylpropan-2-ol |