Online Database of Chemicals from Around the World
| Shenzhen Salubris Pharmaceuticals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (755) 8386-7888 | |||
 |
jtong@salubris.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Zhejiang Chemline industries Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8720-7757 | |||
 |
chemline_08@hotmail.com 360759612@qq.com | |||
| Chemical distributor since 2004 | ||||
| chemBlink standard supplier since 2009 | ||||
| Xinxiang Kaifaqu Chemical Plant | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (373) 507-1644 | |||
 |
sales@xxkfqchem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Wuhan Kemi-works Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8573-6489 | |||
 |
info@kemiworks.net sales@kemiworks.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Selleck Chemicals LLC | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (713) 535-9129 | |||
 |
info@selleckchem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2014 | ||||
| Shanghai Yingrui Biopharm Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3358-5366 3466-6753 +86 13311639313 | |||
 |
sales02@shyrchem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2017 | ||||
| Classification | API >> Antibiotics >> Beta-lactamase inhibitor |
|---|---|
| Name | Meropenem |
| Synonyms | (4R,5S,6S)-3-[[(3S,5S)-5-Dimethylcarbamoylpyrrolidin-3- |
| Molecular Structure | 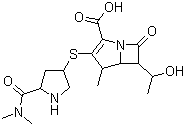 |
| Molecular Formula | C17H25N3O5S |
| Molecular Weight | 383.46 |
| CAS Registry Number | 96036-03-2 |
| EC Number | 641-424-1 |
| SMILES | C[C@@H]1[C@@H]2[C@H](C(=O)N2C(=C1S[C@H]3C[C@H](NC3)C(=O)N(C)C)C(=O)O)[C@@H](C)O |
| Solubility | 77 mg/mL (DMSO), 8 mg/mL (water), <1 mg/mL (ethanol) (Expl.) |
|---|---|
| Density | 1.4±0.1 g/cm3, Calc.* |
| Index of Refraction | 1.639, Calc.* |
| Boiling Point | 627.4±55.0 ºC (760 mmHg), Calc.* |
| Flash Point | 333.2±31.5 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS07;GHS08 Dander Details
GHS07;GHS08 Dander Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H317-H319-H334-H335 Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P233-P260-P261-P264-P264+P265-P271-P272-P280-P284-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P333+P317-P337+P317-P342+P316-P362+P364-P403-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
Meropenem is a broad-spectrum antibiotic belonging to the class of carbapenems, which are a subclass of beta-lactam antibiotics. It is used to treat a wide range of bacterial infections and is particularly effective against Gram-negative and Gram-positive bacteria. Meropenem works by inhibiting bacterial cell wall synthesis, leading to cell death. It is commonly used in hospital settings for treating serious infections caused by multidrug-resistant organisms. The discovery of meropenem dates back to the early 1990s. It was developed by the pharmaceutical company Merck & Co., with the aim of improving upon the limitations of earlier beta-lactam antibiotics. Carbapenems like meropenem are known for their stability against beta-lactamases, enzymes produced by certain bacteria that degrade many other beta-lactam antibiotics. This resistance to beta-lactamases makes meropenem an invaluable option for treating infections caused by resistant strains of bacteria. Meropenem was first introduced for clinical use in the late 1990s. It was specifically developed to combat infections caused by pathogens resistant to other antibiotics, such as those involved in hospital-acquired infections. The unique structure of meropenem, which contains a beta-lactam ring and a sulfur atom, allows it to bind to and inhibit the action of penicillin-binding proteins (PBPs), which are essential for bacterial cell wall synthesis. Without functional PBPs, bacteria are unable to maintain the structural integrity of their cell walls, ultimately leading to cell death. Meropenem has been shown to be effective against a broad spectrum of bacteria, including many that are resistant to other classes of antibiotics. It is used to treat infections such as pneumonia, intra-abdominal infections, urinary tract infections, and bacterial meningitis. Meropenem is also effective against multidrug-resistant organisms such as extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae and carbapenem-resistant Pseudomonas aeruginosa. One of the key advantages of meropenem is its ability to penetrate the blood-brain barrier, making it particularly useful in the treatment of central nervous system infections, including meningitis. The drug is administered intravenously, typically in hospital settings, due to its potent effects and the need for precise dosing in serious infections. Meropenem has become an essential tool in the management of serious and life-threatening infections. Its use, however, has been somewhat limited by concerns over the development of resistance. While meropenem is more resistant to beta-lactamases than other antibiotics, overuse or inappropriate use of the drug can still lead to the emergence of resistance. As a result, efforts have been made to reserve meropenem for cases where other antibiotics are ineffective, to slow the development of resistance. In addition to its use in treating bacterial infections, meropenem is also being studied in combination with other antibiotics to enhance its efficacy and combat the rise of multidrug-resistant bacteria. Researchers are investigating its potential use in treating infections caused by emerging resistant pathogens, and efforts are ongoing to develop new formulations of meropenem that may be more effective against resistant strains. In summary, meropenem is a crucial antibiotic in the treatment of serious bacterial infections, particularly those caused by multidrug-resistant organisms. Its development has significantly improved the outcomes for patients with life-threatening infections, although ongoing vigilance is required to prevent the emergence of resistance. References 2021. Studies on meropenem and cefixime metal ion complexes for antibacterial activity. Future Journal of Pharmaceutical Sciences, 7(1). DOI: 10.1186/s43094-021-00379-0 2009. Rapid Determination of Meropenem in Biological Fluids by LC: Comparison of Various Methods for Sample Preparation and Investigation of Meropenem Stability. Chromatographia, 70(5). DOI: 10.1365/s10337-009-1304-8 2019. Asymmetric Synthesis of a Carbapenem Intermediate. Synfacts, 15(2). DOI: 10.1055/s-0037-1611189 |
| Market Analysis Reports |
| List of Reports Available for Meropenem |