Online Database of Chemicals from Around the World
| Zhenjiang Haitong Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (511) 8448-0369 +86 13306101193 | |||
 |
568344917@qq.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2006 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Ring Specialty Chemicals Inc. | Canada | Inquire | ||
|---|---|---|---|---|
 |
+1 (416) 493-6870 | |||
 |
info@ringchemicals.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hubei Tuoyuan Fine Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (027) 8470-0466 | |||
 |
59741184@qq.com | |||
 |
QQ chat | |||
 |
WeChat: 13260668040 | |||
| Chemical manufacturer since 2025 | ||||
| chemBlink standard supplier since 2011 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| J&H Chemical HK Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8739-6430 8739-6432 | |||
 |
sales@jhechem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink standard supplier since 2023 | ||||
| Classification | Chemical reagent >> Organic reagent >> Carboxylic anhydride |
|---|---|
| Name | Methacrylic anhydride |
| Synonyms | 2-Methyl-2-Propenoic acid anhydride |
| Molecular Structure | 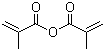 |
| Molecular Formula | C8H10O3 |
| Molecular Weight | 154.16 |
| CAS Registry Number | 760-93-0 |
| EC Number | 212-084-8 |
| SMILES | CC(=C)C(=O)OC(=O)C(=C)C |
| Density | 1.0±0.1 g/cm3 Calc.*, 1.033 g/mL (Expl.) |
|---|---|
| Melting point | -109 ºC (Expl.) |
| Boiling point | 202.3 ºC 760 mmHg (Calc.)*, 225.1 ºC (Expl.) |
| Flash point | 84.4 ºC (Calc.)*, 84 ºC (Expl.) |
| Index of refraction | 1.443 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS05;GHS06;GHS07 Danger Details
GHS05;GHS06;GHS07 Danger Details | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H302-H315-H317-H318-H331-H332-H335 Details | ||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P272-P280-P301+P317-P302+P352-P304+P340-P305+P354+P338-P316-P317-P319-P321-P330-P332+P317-P333+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3265 | ||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||
|
Methacrylic anhydride is a reactive chemical compound widely used as a key reagent in organic synthesis and polymer chemistry. It is the acid anhydride derivative of methacrylic acid, featuring two methacryloyl groups linked by an oxygen atom. The molecular structure allows it to readily react with nucleophiles such as alcohols, amines, and water, making it a versatile acylating agent. Methacrylic anhydride was developed as an alternative to methacrylic acid for introducing methacryloyl functionality into molecules, especially in the synthesis of monomers and polymers. Its ability to transfer methacryloyl groups efficiently under mild conditions has made it a valuable compound in the preparation of methacrylate-based materials. One of the primary applications of methacrylic anhydride is in the modification of natural and synthetic polymers to introduce polymerizable methacrylate groups. For example, it is used to functionalize hydroxyl-containing polymers such as cellulose, chitosan, and polyethylene glycol, enabling the formation of crosslinked networks upon polymerization. This process is fundamental in producing hydrogels, adhesives, coatings, and dental materials with tailored mechanical and chemical properties. In the pharmaceutical and biomedical fields, methacrylic anhydride is employed to synthesize methacrylated derivatives of biomolecules. These modified biomolecules can be polymerized to form biocompatible hydrogels and scaffolds for tissue engineering, drug delivery, and regenerative medicine applications. The anhydride’s reactivity allows for precise control over functionalization, which is critical for tuning the physical and biological characteristics of the resulting materials. Methacrylic anhydride is also important in the manufacture of specialty polymers such as polymethacrylates and copolymers used in paints, adhesives, and plastics. These polymers benefit from the chemical versatility and stability imparted by the methacrylate groups. The compound is typically handled under controlled conditions due to its reactivity and potential to hydrolyze in the presence of moisture, releasing methacrylic acid. Proper storage and handling protocols are essential to maintain its integrity and effectiveness in chemical reactions. In summary, methacrylic anhydride is a crucial reagent for introducing methacryloyl groups into a wide range of molecules, facilitating the synthesis of functional polymers and biomaterials. Its discovery and development have significantly advanced polymer chemistry and biomedical engineering by enabling the creation of materials with customized properties and applications. References 1965. Reaction of 1, 1-disubstituted hydrazines with derivatives of α, β-unsaturated acids Communication 1. Formation of 1, 1-dimethylpyrazolinium 3-oxides. Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science, 14(8). DOI: 10.1007/bf00846199 1991. 13C NMR of Crosslinked Poly(Methacrylic Anhydride). Solid State NMR of Polymers. DOI: 10.1007/978-1-4899-2474-2_10 2024. Fast microwave-assisted methacrylation of Pluronics for photoinduced 3D printing. MRS Communications. DOI: 10.1557/s43579-024-00618-4 |
| Market Analysis Reports |
| List of Reports Available for Methacrylic anhydride |