Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Heta Pharm & Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8829-8691 | |||
 |
sales@hetapharm.com zhb0309@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| NetQem, LLC | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (919) 544-4122 | |||
 |
sales@netqem.us | |||
| Chemical distributor since 2013 | ||||
| chemBlink standard supplier since 2014 | ||||
| Amadis Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8992-5085 | |||
 |
sales@amadischem.com | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2015 | ||||
| Carbosynth China Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 6260-5585 | |||
 |
sales@carbosynth.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2016 | ||||
| Xinxiang Aurora Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (373) 308-8722 +86 18637352520 | |||
 |
info@aurora-biotech.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink standard supplier since 2017 | ||||
| Shanghai Yingrui Biopharm Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3358-5366 3466-6753 +86 13311639313 | |||
 |
sales02@shyrchem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2017 | ||||
| Zhejiang Hisun Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651899693 | |||
 |
yiwen.sun@hisunpharm.com | |||
| Chemical manufacturer since 1956 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | API >> Antineoplastic agents >> Antimetabolite antineoplastic |
|---|---|
| Name | Cytarabine hydrochloride |
| Synonyms | 1-beta-D-Arabinofuranosylcytosine hydrochloride; Cytosine arabinoside hydrochloride |
| Molecular Structure | 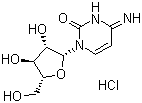 |
| Protein Sequence | N |
| Molecular Formula | C9H13N3O5.HCl |
| Molecular Weight | 279.68 |
| CAS Registry Number | 69-74-9 |
| EC Number | 200-713-9 |
| SMILES | C1=CN(C(=O)N=C1N)[C@H]2[C@H]([C@@H]([C@H](O2)CO)O)O.Cl |
| Solubility | 10 mM (DMSO), 40 mg/ml (CHCl3) (Expl.) |
|---|---|
| Melting point | 197-198 ºC (Expl.) |
| Hazard Symbols |

 GHS07;GHS08 Warning Details
GHS07;GHS08 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H317-H319-H341-H361 Details | ||||||||||||||||||||||||
| Precautionary Statements | P203-P261-P264+P265-P272-P280-P302+P352-P305+P351+P338-P318-P321-P333+P317-P337+P317-P362+P364-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
Cytarabine hydrochloride, also known as Ara-C, is a chemotherapy drug that has been widely used in the treatment of various hematologic malignancies, particularly leukemias. It is a nucleoside analog that mimics the structure of cytosine, a naturally occurring nucleobase in DNA, and was first developed in the 1960s. Cytarabine is a derivative of arabinose, a sugar molecule, and its chemical structure is designed to inhibit the synthesis of DNA, making it effective as an anticancer agent. The discovery of cytarabine hydrochloride was part of a larger effort in the mid-20th century to develop chemotherapeutic agents that could specifically target rapidly dividing cancer cells. Researchers synthesized cytarabine by modifying the sugar moiety of nucleosides, aiming to create a molecule that would be incorporated into the growing DNA chain during cell division, thereby disrupting normal cellular processes. Upon entering the cell, cytarabine is metabolized into its active form, cytarabine triphosphate, which competes with the natural nucleotides for incorporation into DNA during replication. This incorporation leads to chain termination and halts DNA synthesis, ultimately causing cell death. Cytarabine hydrochloride is primarily used in the treatment of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and other hematologic cancers such as non-Hodgkin lymphoma. It is most commonly used in combination with other chemotherapeutic agents as part of aggressive treatment regimens aimed at achieving remission in patients with these cancers. Cytarabine is also employed in high-dose therapy for central nervous system (CNS) involvement in leukemia, where it can be administered intrathecally (directly into the cerebrospinal fluid) to target cancer cells in the brain and spinal cord. Cytarabine’s effectiveness comes with certain limitations and potential side effects. Due to its action on rapidly dividing cells, it not only affects cancer cells but can also damage healthy cells, particularly those in the bone marrow, gastrointestinal tract, and hair follicles. As a result, common side effects include myelosuppression (reduced bone marrow activity), nausea, vomiting, and hair loss. Additionally, high doses of cytarabine can lead to neurotoxicity, including cerebellar toxicity, which can manifest as coordination problems and other neurological issues. Over the years, researchers have explored ways to enhance the efficacy and reduce the side effects of cytarabine. Modifications to its dosing regimens, such as the use of continuous infusion or liposomal formulations, have been developed in an attempt to optimize its therapeutic index. These strategies aim to increase the concentration of the drug in the tumor site while minimizing exposure to normal tissues. In conclusion, cytarabine hydrochloride remains a cornerstone in the treatment of hematologic malignancies. Its ability to inhibit DNA synthesis makes it a powerful tool in cancer therapy, particularly for leukemia. While it is associated with significant side effects, ongoing research continues to improve its use, offering hope for better treatment outcomes in cancer care. References 2024. Mini-consolidations or intermediate-dose cytarabine for the post-remission therapy of AML patients over 60. A retrospective study from the DATAML and SAL registries. American Journal of Hematology, 99(12). DOI: 10.1002/ajh.27510 1983. Arabinosylcytosine. Modes and Mechanisms of Microbial Growth Inhibitors. DOI: 10.1007/978-3-642-68946-8_2 1979. Synthesis and antitumor activity of 1-beta-D-arabinofuranosylcytosine conjugates of cortisol and cortisone. Biochemical and Biophysical Research Communications, 88(4). DOI: 10.1016/0006-291x(79)91110-0 |
| Market Analysis Reports |
| List of Reports Available for Cytarabine hydrochloride |