Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | API >> Antineoplastic agents >> Hormone antineoplastic agents |
|---|---|
| Name | Letrozole |
| Synonyms | 4,4'-(1H-1,2,4-Triazol-1-ylmethylene)bisbenzonitrile |
| Molecular Structure | 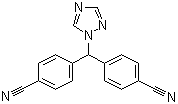 |
| Molecular Formula | C17H11N5 |
| Molecular Weight | 285.31 |
| CAS Registry Number | 112809-51-5 |
| EC Number | 675-034-8 |
| SMILES | C1=CC(=CC=C1C#N)C(C2=CC=C(C=C2)C#N)N3C=NC=N3 |
| Density | 1.1±0.1 g/cm3, Calc.* |
|---|---|
| Melting point | 181-183 ºC |
| Index of Refraction | 1.615, Calc.* |
| Boiling Point | 472.0±55.0 ºC (760 mmHg), Calc.* |
| Flash Point | 214.2±24.5 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS07;GHS08 Dander Details
GHS07;GHS08 Dander Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H351-H360-H373 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P203-P260-P264-P264+P265-P280-P302+P352-P305+P351+P338-P318-P319-P321-P332+P317-P337+P317-P362+P364-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Letrozole, a nonsteroidal aromatase inhibitor, was developed in the early 1990s as a targeted therapy for hormone receptor-positive breast cancer. Initially synthesized to address the need for more effective and selective treatments for estrogen-driven cancers, letrozole works by inhibiting the aromatase enzyme, responsible for converting androgens into estrogens. By blocking this conversion, letrozole significantly reduces circulating estrogen levels, thereby slowing the growth of hormone-dependent tumors. The compound was approved by the U.S. Food and Drug Administration (FDA) in 1997 for use in postmenopausal women with advanced breast cancer. Letrozole's mechanism of action specifically targets the aromatase enzyme, which is essential for estrogen biosynthesis. In postmenopausal women, estrogen production occurs primarily in peripheral tissues, such as adipose tissue, where androgens like testosterone are converted to estrogens by aromatase. By competitively binding to the enzyme's active site, letrozole effectively suppresses estrogen synthesis, making it highly beneficial for estrogen receptor-positive (ER+) breast cancer cases, which account for approximately 70% of all breast cancers. The selectivity of letrozole for aromatase, compared to other steroidal inhibitors, allows for potent inhibition with fewer off-target effects. Letrozole is commonly used in various stages of breast cancer treatment. For early-stage breast cancer, it serves as an adjuvant therapy to prevent recurrence following surgery and chemotherapy. In advanced or metastatic breast cancer, it is employed as a first-line treatment, providing an alternative to traditional chemotherapeutic agents. Clinical trials have demonstrated that letrozole can significantly improve disease-free survival and overall survival rates compared to older therapies, such as tamoxifen. Its favorable safety profile and efficacy have led to widespread adoption in clinical practice. Beyond breast cancer, letrozole has found applications in fertility treatments. It is prescribed off-label for ovulation induction in women with polycystic ovary syndrome (PCOS) who are resistant to clomiphene citrate. Letrozole's ability to lower estrogen levels promotes the release of follicle-stimulating hormone (FSH), thereby stimulating ovulation. Studies have shown that letrozole may lead to higher ovulation and live birth rates compared to other treatments, making it a valuable option in reproductive medicine. Letrozole is also being explored for potential use in other estrogen-related conditions, such as endometriosis and gynecomastia. Research into its pharmacokinetics and structure-activity relationships continues to refine its therapeutic applications and improve patient outcomes. Its discovery marked a significant advance in the field of targeted hormone therapies, offering an effective and less toxic alternative for managing hormone-dependent diseases. The development and success of letrozole underscore the importance of targeted therapies in oncology and beyond. By specifically inhibiting estrogen biosynthesis, letrozole has transformed the treatment landscape for breast cancer and expanded possibilities in reproductive health. References 2005. Aromatase Inhibitors in the Treatment of Breast Cancer. Endocrine Reviews. DOI: 10.1210/er.2004-0015 2005. Aromatase Inhibition: Translation into a Successful Therapeutic Approach. Clinical cancer research : an official journal of the American Association for Cancer Research. DOI: 10.1158/1078-0432.ccr-04-2187 2005. Letrozole-, Anastrozole-, and Tamoxifen-Responsive Genes in MCF-7aro Cells: A Microarray Approach. Molecular cancer research : MCR. DOI: 10.1158/1541-7786.mcr-04-0122 |
| Market Analysis Reports |
| List of Reports Available for Letrozole |