Online Database of Chemicals from Around the World
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Nanjing Hongbaoli Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 5839-0038 | |||
 |
market@hongbaoli.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Jiaxing Jinyan Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (573) 8558-6555 | |||
 |
river.mao@chinadanson.com shanbingjun@jurongchem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| The Dow Chemical Company | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (989) 633-1706 | |||
 |
fmdcigs@dow.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2012 | ||||
| Riverland Trading LLC. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (336).944-2293 | |||
 |
bens@riverlandtrading.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2023 | ||||
| Changzhou Yuping Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8616-0288 8615-8021 | |||
 |
jushun@liyangchem.com | |||
| Chemical manufacturer since 1988 | ||||
| Classification | Biochemical >> Amino acids and their derivatives >> Amino alcohol derivative |
|---|---|
| Name | Diisopropanolamine |
| Synonyms | Bis(2-hydroxypropyl)amine; 1,1'-Iminodi-2-propanol |
| Molecular Structure | 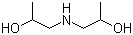 |
| Molecular Formula | C6H15NO2 |
| Molecular Weight | 133.19 |
| CAS Registry Number | 110-97-4 |
| EC Number | 203-820-9 |
| SMILES | CC(CNCC(C)O)O |
| Density | 1.004 g/mL (Expl.) |
|---|---|
| Melting point | 42-45 ºC (Expl.) |
| Boiling point | 249-250 ºC (745 mmHg) (Expl.) |
| Refractive index | 1.4615-1.4635 |
| Flash point | 135 ºC (Expl.) |
| Water solubility | 870 g/L (20 ºC) |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H318-H319 Details | ||||||||||||||||
| Precautionary Statements | P264+P265-P280-P305+P351+P338-P305+P354+P338-P317-P337+P317 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
Diisopropanolamine is an organic compound that belongs to the class of alkanolamines, characterized by the presence of both hydroxyl and amine functional groups. It is a colorless to pale yellow liquid with a mild amine-like odor and is highly soluble in water and organic solvents. The compound is widely used in industrial, pharmaceutical, and agricultural applications due to its amphiphilic nature, which allows it to function as a surfactant, emulsifier, and neutralizing agent. The discovery and utilization of diisopropanolamine have played an essential role in the advancement of chemical formulations and material science. The synthesis of diisopropanolamine involves the reaction of ammonia with propylene oxide, leading to the formation of various isopropanolamines, including mono-, di-, and triisopropanolamine. The production process can be controlled to favor the formation of diisopropanolamine by adjusting reaction conditions such as temperature, pressure, and molar ratios. The compound is commercially available in both pure and mixed forms, with applications spanning multiple industries. One of the most significant uses of diisopropanolamine is in the formulation of metalworking fluids and lubricants. Its ability to neutralize acidic components while maintaining solubility in both water and oil-based systems makes it an ideal additive for cutting fluids, corrosion inhibitors, and emulsifiers used in machining and industrial lubrication. It enhances the stability of formulations, prevents rust formation, and improves the overall performance of metal treatment solutions. In the pharmaceutical industry, diisopropanolamine is utilized as a pH regulator and emulsifier in drug formulations. Its mild basicity allows it to stabilize active pharmaceutical ingredients and enhance the solubility of poorly soluble drugs. It is also used as an intermediate in the synthesis of certain pharmaceuticals, contributing to the production of active compounds with enhanced bioavailability and therapeutic properties. The agricultural sector has also benefited from the properties of diisopropanolamine, particularly in the formulation of herbicides and pesticides. It serves as a neutralizing and solubilizing agent in glyphosate-based herbicides, improving the efficiency of active ingredients in controlling unwanted plant growth. By enhancing the penetration and absorption of herbicidal compounds, diisopropanolamine contributes to the effectiveness of agricultural treatments. Another important application of diisopropanolamine is in gas treatment processes, where it is used to remove acidic gases such as carbon dioxide and hydrogen sulfide from natural gas and industrial emissions. Its ability to selectively absorb acidic components while remaining chemically stable under processing conditions makes it a valuable compound in gas scrubbing systems. This application plays a crucial role in reducing environmental pollution and improving the quality of industrial gas streams. Safety considerations for diisopropanolamine include its potential to cause skin and eye irritation upon direct contact. Proper handling measures, including the use of personal protective equipment and adherence to safety guidelines, are necessary to minimize occupational exposure. While the compound is generally regarded as having low acute toxicity, long-term exposure should be managed to prevent any adverse health effects. Research into the further applications of diisopropanolamine continues, particularly in sustainable and green chemistry initiatives. Efforts are being made to optimize its use in biodegradable formulations, environmentally friendly surfactants, and improved gas treatment technologies. Its versatility and chemical stability ensure that it remains an important compound in various scientific and industrial domains. References 2024. Corrosion in Amine Gas Capturing Facilities: Processes, Challenges, and Mitigations � a Review. Process Integration and Optimization for Sustainability. DOI: 10.1007/s41660-024-00454-y 2021. Magnetic properties of hexanuclear iron(III) cluster containing benzoic acid and amino-alcohol ligand, diisopropanolamine. Magnetic Properties of Paramagnetic Compounds, Magnetic Susceptibility Data, Volume 1. DOI: 10.1007/978-3-662-62478-4_487 2021. Molar magnetic moment of hexanuclear iron(III) cluster containing pivalic acid, diisopropanolamine and amino-alcohol ligands. Magnetic Properties of Paramagnetic Compounds, Magnetic Susceptibility Data, Volume 1. DOI: 10.1007/978-3-662-62478-4_486 |
| Market Analysis Reports |
| List of Reports Available for Diisopropanolamine |